DOI: https://doi.org/10.34069/AI/2024.81.09.11
How to Cite:
Bobrova, M., Gencheva, V., Holodaieva, O., Myhal, I., & Tsviakh, O. (2024). Effect of storage time on antioxidant content in seeds of agricultural plants. Amazonia Investiga, 13(81), 142-156. https://doi.org/10.34069/AI/2024.81.09.11
Effect of storage time on antioxidant content in seeds of agricultural plants
Вплив тривалості зберігання на вміст антиоксидантів у насінні сільськогосподарських рослин
Received: July 30, 2024 Accepted: September 15, 2024
Written by:
Bobrova Mariia
https://orcid.org/0000-0001-7703-651X
WoS Researcher ID: GLN-6580-2022
Ph.D., in Biology, Associate Professorof the Department of Natural Sciences and their teaching methods of the Volodymyr Vynnychenko Central Ukrainian State University, Kropyvnytskyi, Ukraine.
Gencheva Viktoriia
https://orcid.org/0000-0002-8764-4559
WoS Researcher ID: LMQ-5323-2024
Candidate of biological sciense, Associate Professor of the Department of Chemistry, Zaporizhzhia National University, Zaporizhzhia, Ukraine.
Holodaieva Olena https://orcid.org/0000-0002-4922-7033
WoS Researcher ID: ABE-3903-2020
Ph.D., in Chemistry, Associate Professor of the Department of of the Fundamental Disciplines of the International European University, Kyiv, Ukraine.
Myhal Ivan
https://orcid.org/0000-0002-9786-538X
WoS Researcher ID: LNP-6091-2024
Ph.D., in Medicine, Researcher at the Department of Extracorporeal Hematology and Stem Cell Transplantation of State institution "Institute of Blood Pathology and Transfusion Medicine of the National Academy of Medical Sciences of Ukraine", Lviv, Ukraine.
Tsviakh Olha
https://orcid.org/0000-0002-1119-2170
WoS Researcher ID: AAB-5503-2022
Ph.D., in Biology, Senior Lecturer of the Department of Physical Education and Sport of the V.O. Sukhomlynskyi Mykolaiv National University, Mykolaiv, Ukraine.
Abstract
This study investigated the impact of storage time on the prooxidant-antioxidant balance (PAB) in seed tissues of 12 agricultural plant species, including both monocots and dicots. We measured superoxide generation, TBA-active products, and the activity of enzymatic antioxidants (superoxide dismutase, catalase, and cytochrome oxidase) and non-enzymatic antioxidants (ascorbic acid and glutathione). Biochemical parameters were recorded monthly for one year.
Our results demonstrated that the activity of enzymatic antioxidants and the content of non-enzymatic antioxidants decreased with increased seed storage time. Conversely, both the generation of reactive oxygen species and the level of free radical damage to biomolecules increased. The percentage change in free radical peroxidation and antioxidant protection depended on the initial PAB status of the seeds. Monocots exhibited a greater overall increase in prooxidant activity during storage, while dicots showed a less pronounced decrease in antioxidant content. A notable surge in prooxidant activity and a corresponding decline in antioxidant activity occurred at 9-10 months of storage for dicots and 6-7 months for monocots. These findings highlight the importance of considering storage time and species-specific differences to optimize planting strategies and seed care, as well as the use of appropriate plant foods.
Keywords: prooxidants, antioxidants, ascorbic acid, catalase.
Анотація
У цьому дослідженні вивчався вплив терміну зберігання на прооксидантно-антиоксидантний баланс (ПАБ) у тканинах насіння 12 видів однодольних та дводольних сільськогосподарських рослин. Визначали рівень генерації супероксиду, вміст ТБК-активних продуктів, активність ферментативних (супероксиддисмутази, каталази, цитохромоксидази) та низькомолекулярних антиоксидантів (аскорбінової кислоти та глутатіону). Біохімічні показники реєстрували щомісяця протягом року.
У результатах наших досліджень показано, що активність ферментативних антиоксидантів і вміст низькомолекулярних антиоксидантів знижувалися зі збільшенням терміну зберігання насіння. Тоді ж як утворення активних форм Оксигену та вільнорадикальне пошкодження біомолекул зросли. Відсоткова зміна вільнорадикального перекисного окислення та антиоксидантного захисту залежала від початкового ПАБ-статусу насіння. Дослідні однодольні рослини показали більше загальне збільшення прооксидантної активності під час зберігання, тоді як дводольні продемонстрували менш виражене зниження вмісту антиоксидантів. Помітний стрибок прооксидантної активності та відповідне зниження антиоксидантного захисту спостерігався на 9-10 місяці зберігання для дводольних та на 6-7 місяці для однодольних. Ці висновки підкреслюють важливість урахування часу зберігання та видоспецифічних відмінностей для оптимізації стратегії посадки та догляду за насінням, а також вживання відповідних рослинних продуктів харчування.
Ключові слова: прооксиданти, антиоксиданти, аскорбінова кислота, каталаза.
Introduction
Antioxidants (AOs) are biologically active natural protectors of our body that promote adaptation to stressful conditions and changing environment, accompany normal growth and development processes, inhibit aging processes, promote regeneration and recovery from diseases and disorders, help to get out of the prodromal period into the state of homeostasis, and are natural antimutational agents (Marrocco et al., 2017; Kohen & Nyska, 2002; Halliwell, 2006). Our body synthesises its own AOs and replenishes their reserves from plant and animal origin products, and the content of AOs even in the healthiest food significantly depends on the conditions and duration of the storage (Xu et al., 2017; Song et al., 2010; Shao et al., 2008). A decrease in the content of AOs in the body leads to an increase in the amount of prooxidants (POs), represented by reactive Oxygen species (ROS), other free radicals and their transformation products (Pacheco et al., 2018). According to numerous scientific studies, the primary cause of diseases at the molecular level is damage to biopolymers. For example, ROS cause the formation of free radicals that trigger chain reactions of protein damage, the creation of interstrand cross-links, which makes DNA incapable of transcription and replication, and, in turn, makes normal cell division and protein biosynthesis impossible (Scandalios, 2002; Van Breusegem & Dat, 2006). Damage to the integrity of cell organelle membranes and plasma membrane is the first cytological indicator of most diseases (Dickinson, 2003). Free radicals cause peroxidation of membrane lipids, creation of intermolecular cross-links of fatty acid fragments, which changes the balance of membrane viscosity and fluidity and disrupts its transport properties. An increase in the content of free radicals leads to the destruction of biologically active substances synthesised by our body and obtained from food, which leads to a decrease in the nutritional value of food, its metabolic capacity, and therefore its benefits (Rampon et al., 2018; Rhoads et al., 2006; Apel & Hirt, 2004; Foyer & Noctor, 2009; Janků et al., 2019; Mittler, 2017). The imbalance of the prooxidant-antioxidant system (PAS) in seed tissues leads to a decrease in germination, which in turn leads to unnecessary costs for seed procurement, irrational use of sown areas and extensive farming (Bartoli et al., 2013; Oracz & Karpinski, 2016; Kumar et al., 2011) to take into account the storage time of plant products when planning a diet. For example, our previous studies have shown that soaking seeds leads to the initiation of germination processes and an increase in the level of AOs, but plant products that have been stored for a long time may contain very low baseline levels of AOs or not contain them at all. This devalues the benefits of whole grain products and so-called "live cereals", in the preparation of which pre-soaking and minimal heat treatment is recommended in order to preserve the maximum amount of biologically active substances in food. All of the above-mentioned enhances the relevance of the research topic and its significant practical importance for a wide range of readers and consumers.
The objective is to identify patterns of changes in the prooxidant-antioxidant balance in tissues depending on the storage time of plant products.
To achieve the objective, we identified the following tasks:
2) To determine the change in the content of low molecular weight AOs in plant tissues, depending on the storage period;
3) To experimentally confirm the change in the content of POs in plant tissues, depending on the storage period;
4) To determine the change in the content of free radical peroxidation products (FRP) of membranes in plant tissues, depending on the storage period;
5) To investigate changes in the activity of membrane markers of free radical peroxidation in plant tissues, depending on the storage period;
6) To trace the change in the balance of PAS links depending on the storage period of plant products;
7) To determine the species-specific features of the PAS state of experimental plants.
The article consists of the review of the literature, which provides an analysis of the works of advanced scientists in this direction, and highlights the components that require further research and systematization. In the methodology section, the principles of constructing the research scheme are given, the expediency of the choice of methods of analysis of each component of the state of the prooxidant-antioxidant system is substantiated, the conditions and repetition of the experiment are indicated. The analysis and generalization of the research results is maximally illustrated by graphs that reflect the dynamics of changes in the value of research indicators every month during the year, which is convenient for perception, visualizes the interspecies difference, and serves as a basis for conclusions, practical recommendations and prospects for further scientific research.
Literature Review
A number of leading scientists have studied the importance of POs and AOs (Halliwell, 2006; Shao et al., 2008; Pacheco et al., 2018; Scandalios, 2002; Apel & Hirt, 2004; Foyer & Noctor, 2009; Janků et al., 2019; Mittler, 2017). One of the top largest biochemical schools that regularly works in this area is the school of Nicholas Smirnoff (Smirnoff, 2005, 2019). Numerous achievements in PAS biochemistry in Ukraine are made by O.P. Dmytriyev, Z.M. Kravchuk, Y.E. Kolupaev, Y.V. Karpets, (Dmytriyev & Kravchuk, 2005; Kolupaev et al., 2019). Most scientists agree that the main enzymatic AOs are superoxide dismutase (SOD) (Вerwal & Ram, 2019) and catalase, and low-molecular weight ascorbic acid (AA) and glutathione (GSH). The AOs properties of SOD are described in the works of Baiano A., del Nobile M.A., Вerwal M.K., & Ram C. (Baiano & Nobile, 2016; Вerwal & Ram, 2019), and catalase in the works of Nandi, A., Yan, L. J., Jana, C. K., & Das. (Nandi et al., 2019). The protective role of AA was investigated by Rietjens I.M., Boersma M.G., Haanm Ld., Spenkelink B., Awad H.M., Cnubben N.H., Padayatty S.J., Katz A., Wang Y., Eck P., Kwon O., Lee J.H., Chen S., Corpe C., Levine M., Dutta A., Paciolla S.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis S. (Rietjens et al., 2002; Padayatty et al., 2003; Paciolla et al., 2019).
Szalai G, Kellos T, Galiba G, Kocsy G, Hasanuzzaman M, Nahar K, Anee T.I., Fujita M., experimentally confirmed the role of GSH as AOs (Szalai et al., 2009; Hasanuzzaman et al., 2017, 2019). According to Gautam V., Kaur R., Kohli S.K., Verma V., Kaur P., Singh R., Saini P., Arora S., Thukral A.K., Karpets Yu, Bhardwaj R. the first PO that occurs in a plant cell as a by-product of photosynthesis is singlet oxygen, which is converted to superoxide anion radical (•O2-) (Gautam et al., 2017). The target of •O2- is cell membranes, which as a result of FRPO, form malondialdehyde (MDA) and other TBA-active products (Morales and Munné-Bosch, 2019). A marker of membrane damage is the change in cytochrome oxidase activity, the significance of which is described by Wikström (Wikström et al., 2018). The balance between the formation and POs, and protective effect of AOs is PAS, which at the molecular level responds to the impact of any factors on the body's homeostasis (Dat et al., 2000; Dickinson, 2003; Gill and Tuteja, 2010; Huang & Guo, 2005).
The role of antioxidant in seed quality is described in Mahalingam Govindaraj′s work (Govindaraj et al., 2017), аntioxidant activity and phenolic content of selected fruit seeds is described by Yean-Yean Soong and Philip J Barlow (Soong & Barlow, 2004). Identification and quantification of polyphenols in hull, bran and endosperm of common buckwheat is shown in Zhang′s article (Zhang et al., 2017). Pang and his colleagues investigated the вound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice (Pang et al., 2018). Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars is shown in Kiprovski′s article (Kiprovski et al., 2015). There is no systematic material describing the effect of storage duration on the content of antioxidants in the seeds of agricultural plants, which increases the relevance of our research.
Methodology
Seed tissues of the following plants were the object of experimental studies: Glycine max L., Helianthus annuus L., Fagopyrum esculentum L., Linum usitatissimum L., Sinapis alba L., Chenopodium quinoa L., Panicum miliaceum L., Oryza sativa L., Avena sativa L., Zea mays L., Hordeum vulgare L, Triticum durum Desf. The choice of plant species is due to the popularity of their use in the daily diet and the recommendation of nutritionists regarding a healthy diet.
To create the research scheme, we were guided by the fact that the first and main prooxidant, which is formed in the cells of all living beings in response to stressors, is the superoxide anion radical. A marker of the strengthening of the prooxidant link is its effect on lipid membranes with their subsequent peroxidation and the formation of TBA-active products. The main enzymatic antioxidants of cells are superoxide dismutase and catalase, and low-molecular ones - ascorbic acid and glutathione. The effectiveness of maintaining the prooxidant-antioxidant balance is assessed by the activity of cytochrome oxidase. So, by experimentally investigating the changes in the above indicators, it is possible to draw a conclusion about the state of the prooxidant-antioxidant system, and in our case to draw conclusions about the degree of benefit of the selected food products.
To quantify the change in the value of PAS indicators, we used generally accepted classical methods described in detail in our previous works (Bobrova et al., 2020, 2021, 2022). Thus, the baseline level of •O2- generation was determined using the spectrophotometric nitroblue tetrazolium recovery test (NBT test), the advantage of which is high accuracy and the possibility of determining both the basic level of superoxide generation and its sources, namely mitochondrial, microsomal or cytoplasmic. The chosen method for determining TBA-active products includes pre-incubation of the homogenate in a prooxidant ferrum-ascorbinate buffer with longer photometry, which allows not only to determine the content of malondialdehyde, but also to draw a conclusion about the general degree of free radical damage to membrane lipids. To assess the change in SOD activity, the percentage of inhibition of the oxidation of •O2-adrenaline into adrenochrome was determined, and catalase was determined by titration with potassium permanganate solution, which are classical generally accepted biochemical techniques. The content of AА was determined according to Tillmans titrimetry, and the concentration of GSH was determined by the Elman method, which do not require lengthy sample preparation, combine accuracy and ease of execution, allow selective determination of experimental components with maximum preservation of their nativeness. Cytochrome oxidase activity was determined spectrophotometrically. The peculiarity of the technique is extremely strict observance of the conditions for the preservation of cytochrome to prevent its oxidation. Biochemical parameters were measured monthly for 1 year. All experiments were carried out under standard conditions (air temperature 20 degrees Celsius and absolute pressure 760 mmHg, air humidity 50%). Each control and experimental group included 10 samples when determining each indicated indicator.
Results and Discussion
The results of the study were statistically calculated according to generally accepted methods, the reliability was confirmed at p < 0.05. The repetition of samples for each indicator given in the table is 10. The laboratory analysis of all mentioned indicators was carried out in strict accordance with the order and conditions of conducting the experiment specified in the methods. All experiments were conducted under standard conditions (air temperature 20 degrees Celsius and absolute pressure 760 mmHg, air humidity 50%). The seeds were stored under standard conditions and without access to light. This minimizes potential sources of error and enables the generalization of the results.
For ease of calculation and better clarity of the digital data, we present the baseline level of POs and AOs in the tissues (Tables 1 and 2). In our previous work, we investigated the effect of germination initiation on the prooxidant-antioxidant balance and determined the baseline values of PAS state indicators that are species-specific. Using these results, we set up two experimental lines in parallel: the first involved the initiation of the seed germination process, which is reflected in our 2022 publication (Bobrova et al., 2022). The second direction, the results of which this article is devoted to, included a change in the values of all starting indicators depending on the duration of seed storage, which we fixed every month during the year. The common point of intersection of these two directions of scientific research is the starting level of indicators of the state of PAS, so we consider it appropriate to present in this publication the results of laboratory studies of the basic level of PAS established by us earlier. This need is also justified by the fact that we calculated the results of monthly changes in the values of PAS status indicators as a percentage of the base level, which is the most convenient way to summarize the results on one graph for indicators with different measurement units.
Table 1.
Results of identification of prooxidant activity and the level of FRPO in the inactive seed tissues
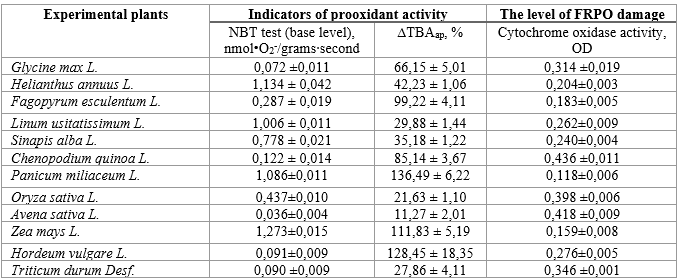
Source: compiled by the authors based on a previous publication (Bobrova, et al., 2022).
Table 2.
Results of identification of antioxidant activity in the inactive seed tissues
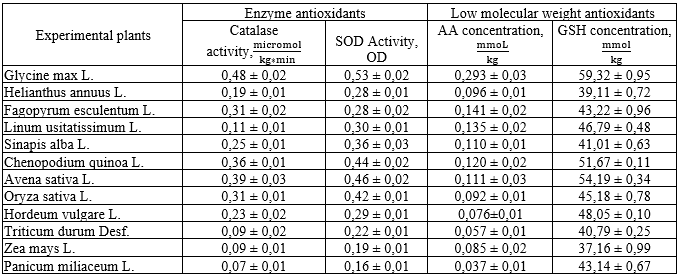
Source: compiled by the authors based on a previous publication (Bobrova, et al., 2022).
Analysing the results obtained, it can be stated that soybean seeds have the lowest baseline level of PO activity, the highest content of both enzymatic and low-molecular weight AOs, therefore the lowest percentage of increase in •O2- and the lowest percentage of decrease in the content of SOD and catalase (Fig. 1). The relatively stable level of GSH is noteworthy.
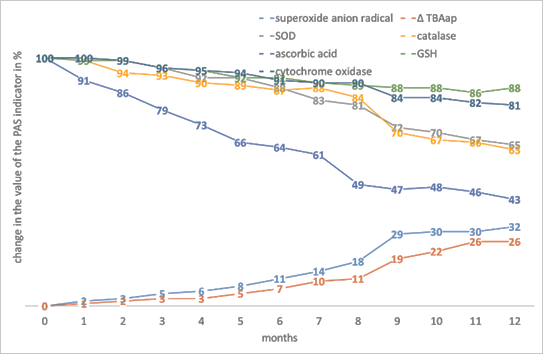
Figure 1. The effect of storage time on the change in the value of the PAS indicators in the tissues of Glycine max L seeds.
A similar pattern was found in the tissues of quinoa seeds (Fig. 2):
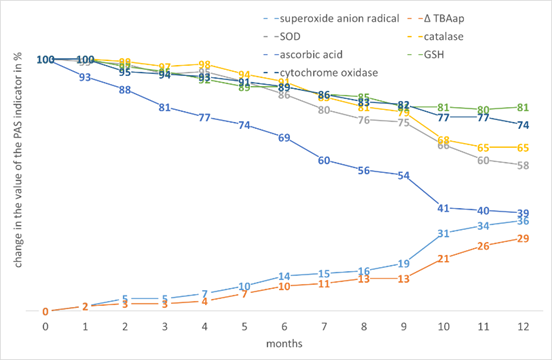
Figure 2. The effect of storage time on the change in the value of the PAS indicators in Chenopodium quinoa L. seed tissues.
A characteristic feature of buckwheat was a relatively stable level of AA, the smallest increase in ∆TBAap and the smallest decrease in cytochrome oxidase, a possible explanation for this is the high content of essential amino acids, potassium, magnesium and iron, which is part of cytochrome (Fig. 3).
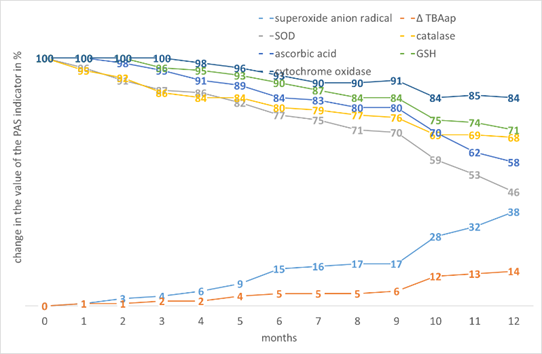
Figure 3. The effect of storage time on the change in the value of the PAS indicators in the tissues of Fagopyrum esculentum L. seeds.
A characteristic feature of flax was one of the lowest increases in ∆TBAap with a fairly high increase in •O2-, which may be explained by the presence of polyunsaturated fatty acids (PUFA) (Fig. 4).
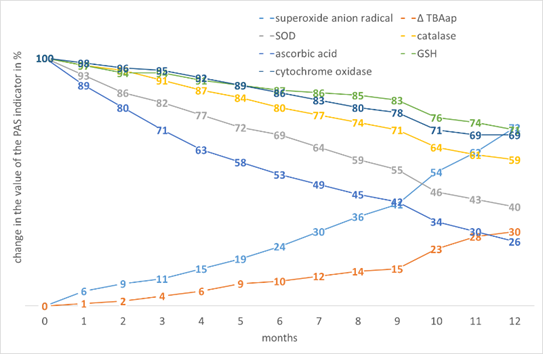
Figure 4. The effect of storage time on the change in the value of the PAS indicators in Linum usitatissimum L. seed tissues.
Among dicotyledons, sunflower has the highest percentage of increase in PO activity with increasing storage time, which is explained by a rather high initial level of •O2- generation (Fig. 5). The decrease in the content of enzymatic and low molecular weight AOs is also the largest, since their initial level was the lowest among all the experimental samples of dicotyledonous seed tissues.
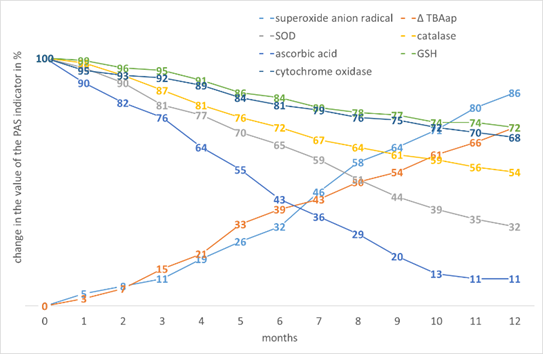
Figure 5. The effect of storage time on the change in the value of the PAS indicators in the tissues of Helianthus annuus L. seeds.
In the tissues of buckwheat, mustard (Fig. 6) and flax, we observe intermediate values of the increase in PO activity and a decrease in the content of AOs with an increase in storage time.
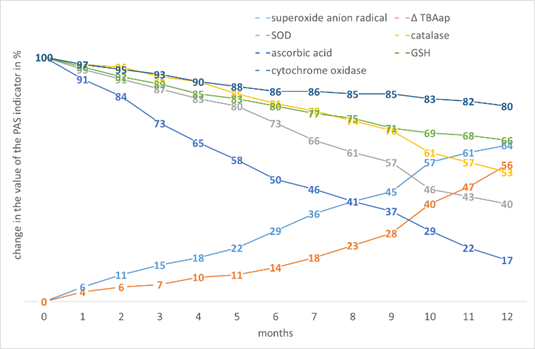
Figure 6. The effect of storage time on the change in the value of the PAS indicators in Sinapis alba L. seed tissues.
Thus, we obtain a tendency of dependence of the percentage of increase in the level of FRPO and decrease in AO protection with an increase in the storage time of tissues on the value of the initial level of PAS indicators.
Among monocotyledons, oats have the highest rates of preservation of AO properties with increasing seed storage time (Fig. 7). It also has the lowest increase of •O2- and ∆TBAap.
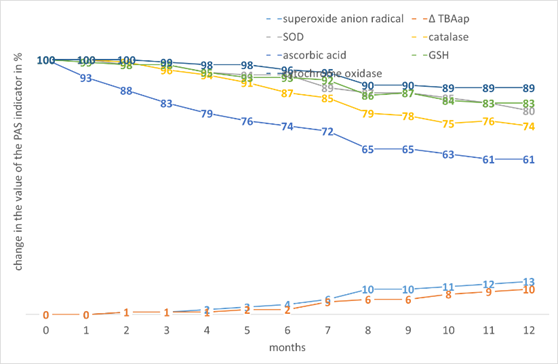
Figure 7. The effect of storage time on the change in the value of the PAS indicators in Avena sativa L. seed tissues.
The worst results are observed for maize. However, it is interesting that with the greatest increase in •O2- and ∆TBAap, the greatest decrease in AO, the cytochrome oxidase activity is quite high, possibly due to the involvement of β-carotenes in stabilisation (Fig. 8).
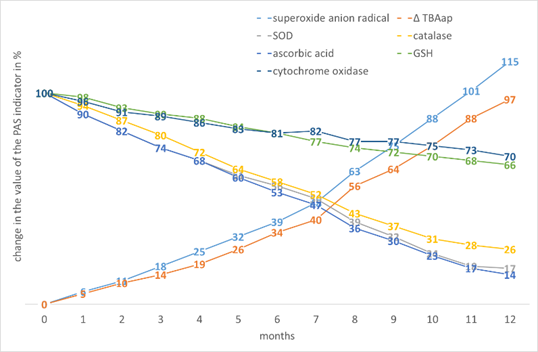
Figure 8. The effect of storage time on the change in the value of the PAS indicators in Zea mays L. seed tissues.
Millet has similar changes in the values of indicators to maize. However, the decline in non-enzymatic AOs is not as intense, however, taking into account their initial low level. The stability of GSH stands out against this background. Millet is also characterised by the largest decrease in cytochrome oxidase activity (Fig. 9).
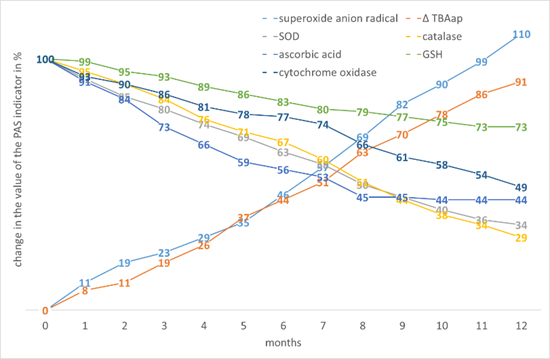
Figure 9. The effect of storage time on the change in the value of the PAS indicators in the tissues of Panicum miliaceum L. seeds.
A characteristic feature of rice is a significant decrease in the intensity of the decline in the level of AOs after 6-7 months of storage. Stable decrease of GSH and intermediate indicators of PO activity between the previously described monocots (Fig. 10).
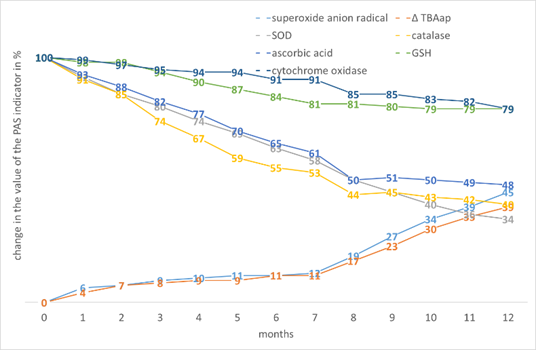
Figure 10. The effect of storage period on the change in the value of the PAS indicators in Oryza sativa L. seed tissues.
Indicators of wheat and barley show similarities in the change of PAS values (Fig. 11, Fig. 12, respectively).
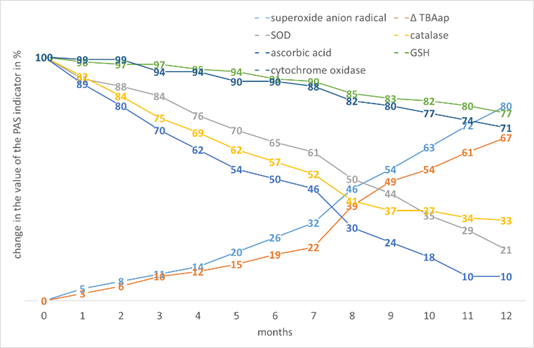
Figure 11. The effect of storage time on the change in the value of the PAS indicators in Triticum durum Desf.
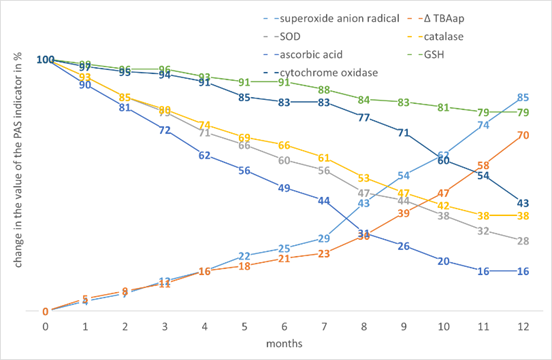
Figure 12. The effect of storage time on the change in the value of the PAS indicators in Hordeum vulgare L. seed tissues.
Summing up all the above, we have identified a pattern according to which an uprise in the growth of PO activity and a corresponding decrease in AO activity, which occurs at 9-10 months of storage, is characteristic of the seed tissues of all dicotyledonous experimental samples, while for monocotyledons this uprise occurs at 6-7 months, which indicates an increased sensitivity of seeds to changes in environmental factors and the effects of stress factors. This must be taken into account to ensure the optimal time for planting and caring for seeds.
GSH does not follow this pattern, it has a stable decrease in content, but in no case does it decrease below 60 %, which may indicate its leading role.
It was observed that the overall increase in PO activity with increasing storage time is higher in monocots, and the decrease in AO content is lower in dicots. This may be explained by the quantitative and qualitative composition of cellular inclusions, which perform not only reserve, trophic, but also protective functions. For example, PUFAs in flax, mustard and sunflower tissues are free radical protectors, and the increased content of protein inclusions in soybean and quinoa tissues is a source of amino acids in the synthesis of enzymatic AOs. The valuable biochemical composition of buckwheat, with its high content of arginine, lysine, cystine, histidine, phosphoric acid, potassium, magnesium and iron, promotes the synthesis of both enzymatic and low-molecular weight AOs.
Conclusion
The prospect of further scientific research is the study of changes in the content of antioxidants, free radicals and their transformation products in the edible parts of fruits and vegetables, as well as the search for ways to preserve their antioxidant activity. It is of great practical importance in nutrition, food hygiene and increased relevance among supporters of a healthy lifestyle.
Bibliographic References
Apel, K., & Hirt, Н. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55(1), 373-399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Baiano, A., & Del Nobile, M. A. (2016). Antioxidant Compounds from Vegetable Matrices: Biosynthesis, Occurrence, and Extraction Systems. Critical Reviews in Food Science and Nutrition, 56(12), 2053-2068. https://doi.org/10.1080/10408398.2013.812059
Bartoli, C. G., Casalongueb, C. A., Simontacchia, M., Marquez-Garciac, B., & Foyer, C. H. (2013). Interactions between a hormone and redox signaling pathways in the control of growth and cross-tolerance to stress. Environmental and Experimental Botany, 94, 73-88. http://dx.doi.org/10.1016/j.envexpbot.2012.05.003
Вerwal, M.K., & Ram, C. (2019). Superoxide Dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. Open access peer-reviewed chapter. DOI: https://doi.org/10.5772/intechopen.82079
Bobrova, M., Holodaieva, O., Koval, S., Kucher, O., & Tsviakh, O. (2021). The effect of hypothermia on the state of the prooxidant-antioxidant system of plants. Revista de la Universidad del Zulia, 12(33), 82-101. https://doi.org/10.46925//rdluz.33.07
Bobrova, M., Holodaieva, O., Arkushyna, H., Larycheva, O., & Tsviakh, O. (2020). The value of the prooxidant-antioxidant system in ensuring the immunity of plants. Revista de la Universidad del Zulia, 11(30), 237-266. https://doi.org/10.46925//rdluz.30.17
Bobrova, M., Holodaieva, O., Koval, S., Kucher, O., & Tsviakh, O. (2022) Features of changes in prooxidant-antioxidant balance of tissues during activation of seed germination. Journal of the University of Zulia, 13(37), 362-382. https://doi.org/10.46925//rdluz.37.23
Dat, J., Vandenabeele, S., Vranova, E. V. M. M., Van Montagu, M., Inzé, D., & Van Breusegem, F. (2000). Dual action of the active oxygen species during plant stress responses. Cellular and Molecular Life Sciences CMLS, 57, 779-795.
Dickinson M. (2003). Molecular plant pathology. London, New York: BIOS Scientific Publishers, 273 р. https://doi.org/10.4324/9780203503300
Dmytriyev O.P., & Kravchuk, Z.M. (2005). Active forms of oxygen and immunity of plants. Cytology and genetics, 39(4), 64-75. http://dspace.nbuv.gov.ua/handle/123456789/126766
Foyer C. H., & Noctor G. (2009). Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants & redox signaling, 11(4), 861-905. https://doi.org/10.1089/ars.2008.2177
Gautam, V., Kaur, R., Kohli, S.K., Verma, V., Kaur, P., Singh, R., Saini, P., Arora, S., Thukral, A.K., Karpets, Yu.V., Kolupaev, Yu.E., & Bhardwaj, R. (2017). ROS compartmentalization in plant cells under abiotic stress condition. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Singapore: Springer, pp. 89-114.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909-930. https://doi.org/10.1016/j.plaphy.2010.08.016
Govindaraj, M., & Poomaruthai, M., & Albert, A. (2017). Role of antioxidant in seed quality-а review. Agricultural Reviews, 38, 180-190. DOI: 10.18805/ag.v38i03.8977
Halliwell, B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant physiology, 141(2), 312-322. https://doi.org/10.1104/pp.106.077073
Hasanuzzaman, M., Nahar, K., Anee, T.I., & Fujita, M. (2017). Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiology and molecular biology of plants, 23, 249-268. https://doi.org/10.1007/s12298-017-0422-2
Hasanuzzaman, M., Bhuyan, M. B., Anee, T. I., Parvin, K., Nahar, K., Mahmud, J. A., & Fujita, M. (2019). Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants, 8(9), 384. https://doi.org/10.3390/antiox8090384
Huang, M., & Guo, Z. (2005). Responses of antioxidant system to chilling stress in two rice cultivars differing in sensitivity. Biologia plantarum, 49, 81-84. https://doi.org/10.1007/s00000-005-1084-3
Janků, М., Luhová, L., & Petřivalský, М. (2019). On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants, 8(4), 105. https://doi.org/10.3390/antiox8040105
Kiprovski, B., Mikulic-Petkovsek, M., Slatnar, A., Veberic, R., Stampar, F., Malencic, D., & Latkovic, D. (2015). Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem, 185, 41-47. https://doi.org/10.1016/j.foodchem.2015.03.137
Kohen, R., & Nyska, A. (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicologic Pathology, 30(6), 620-650. https://doi.org/10.1080/01926230290166724
Kolupaev, Yu.E., Karpets, Yu.V., & Kabashnikova, L.F. (2019) Antioxidative system of plants: cellular compartmentalization, protective and signaling functions, mechanisms of regulation. Applied Biochemistry and Microbiology, 55(5), 441-459. https://doi.org/10.1134/S0003683819050089
Kumar S., Malik J., Thakur P., Kaistha S., Sharma K.D., Upadhyaya H.D. ... & Nayyar, H (2011) Growth and metabolic responses of contrasting chickpea (Cicer arietinum L.) genotypes to chilling stress at reproductive phase. Acta physiologiae plantarum, 33, 779-787. https://doi.org/10.1007/s11738-010-0602y Marrocco, I., Altieri, F., & Peluso, I. (2017). Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative medicine and cellular longevity, 2017(1), 6501046. https://doi.org/10.1155/2017/6501046
Mittler, R. (2017). ROS Are Good. Trends in plant science, 22(1), 11-19. https://doi.org/10.1016/j.tplants.2016.08.002
Morales, M., & Munné-Bosch, S. (2019). Malondialdehyde: Facts and Artifacts. Plant physiology, 180(3), 1246-1250. https://doi.org/10.1104/pp.19.00405
Nandi, A., Yan, L. J., Jana, C. K., & Das, N. (2019). Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative medicine and cellular longevity, 2019(1), 9613090. https://doi.org/10.1155/2019/9613090
Oracz, K., & Karpinski, S. (2016). Phytohormones Signaling Pathways and ROS Involvement in Seed Germination. Frontiers in Plant Science, 7, 864. https://doi.org/10.3389/fpls.2016.00864
Pacheco, J. H. L., Carballo, M. A., & Gonsebatt, M. E. (2018). “Antioxidants against environmental factor-induced oxidative stress,” in Nutritional Antioxidant Therapies: Treatments and Perspectives, K. H. Al-Gubory, Ed., vol. 8, pp. 189-215. Cham, Switzerland: Springer. https://doi.org/10.1007/978-3-319-67625-8
Paciolla, С., Fortunato, S., Dipierro, N., Paradiso, A., & De Leonardis, S. (2019). Vitamin C in Plants: From Functions to Biofortification. Antioxidants, 8(11), 519. https://doi.org/10.3390/antiox8110519
Padayatty, S. J., Katz, A., Wang, Y., Eck, P., Kwon, O., Lee, J. H., … Levine, M. (2003). Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. Journal of the American College of Nutrition, 22(1), 18-35. https://doi.org/10.1080/07315724.2003.10719272
Rampon, C., Volovitch, M., Joliot, A., & Vriz, S. (2018) Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants, 7(11), 159. https://doi.org/10.3390/antiox7110159
Pang, Y., Ahmed, S., Xu, Y., Beta, T., Zhu, Z., Shao, Y., & Bao, J. (2018). Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem, 240, 212-221. https://doi.org/10.1016/j.foodchem.2017.07.095
Rhoads, D. M., Umbach, A. L., Subbaiah, C. C., & Siedow, J. N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant physiology, 141(2), 357-366. https://doi.org/10.1104/pp.106.079129
Rietjens, I. M., Boersma, M. G., de Haan, L., Spenkelink, B., Awad, H. M., Cnubben, N. H., ... & Koeman, J. H. (2002) The prooxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environmental toxicology and pharmacology, 11(3-4), 321-333. https://doi.org/10.1016/s1382-6689(02)00003-0
Scandalios, J.G. (2002). The rise of ROS. Trends in biochemical sciences, 27(9), 483-486. https://doi.org/10.1016/S0968-0004(02)02170-9
Shao, H. B., Chu, L. Y., Shao, M. A., Jaleel, C. A., & Hong-mei, M. (2008). Higher plant antioxidants and redox signaling under environmental stresses. Comptes rendus. Biologies, 331(6), 433-441. https://doi.org/10.1016/j.crvi.2008.03.011
Smirnoff, N. (2005) Antioxidants and reactive oxygen species in plants (pp. 169-177). Oxford: Blackwell.
Smirnoff, N., & Arnaud, D. (2019) Hydrogen peroxide metabolism and functions in plants. New phytologist, 221(3), 1197-1214. https://doi.org/10.1111/nph.15488
Song, W., Derito, C.M., Liu, M.K., He, X., Dong, M., & Liu, R.H. (2010) Cellular antioxidant activity of common vegetables. Journal of Agricultural and Food Chemistry, 58(11), 6621-6629. https://doi.org/10.1021/jf9035832
Szalai, G., Kellos, T., Galiba, G., & Kocsy, G. (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. Journal of Plant Growth Regulation, 28, 66-80. https://link.springer.com/article/10.1007/s00344-008-9075-2
Van Breusegem, F., & Dat, J. (2006) Reactive oxygen species in plant cell death. Plant physiology, 141(2), 384-390. https://dx.doi.org/10.1104%2Fpp.106.078295
Wikström, M., Krab, K., & Sharma, V. (2018). Oxygen activation and energy conservation by cytochrome c oxidase. Chemical reviews, 118(5), 2469-2490. https://doi.org/10.1021/acs.chemrev.7b00664
Xu, D. P., Li, Y., Meng, X., Zhou, T., Zhou, Y., Zheng, J., ... & Li, H. B. (2017). Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. International journal of molecular sciences, 18(1), 96. https://doi.org/10.3390/ijms18010096
Yean-Yean, S., & Barlow, P.J. (2004) Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry, 88(3), 411-417. https://doi.org/10.1016/j.foodchem.2004.02.003
Zhang, W., Zhu, Y., Liu, Q., Bao, J., & Liu, Q. (2017). Identification and quantification of polyphenols in hull, bran and endosperm of common buckwheat (Fagopyrum esculentum) seeds. Journal of Functional Foods, 38, 363-369. https://doi.org/10.1016/j.jff.2017.09.024
https://amazoniainvestiga.info/ ISSN 2322-6307

This article is licensed under the Creative Commons Attribution 4.0 International License (CC BY 4.0). Reproduction, distribution, and public communication of the work, as well as the creation of derivative works, are permitted provided that the original source is cited.